 Close Topic Options
Close Topic OptionsMerck Molnupiravir
Merck Molnupiravir - Psychology, Special Needs, Health - Posted: 24th Dec, 2021 - 3:41pm
Merck Molnupiravir
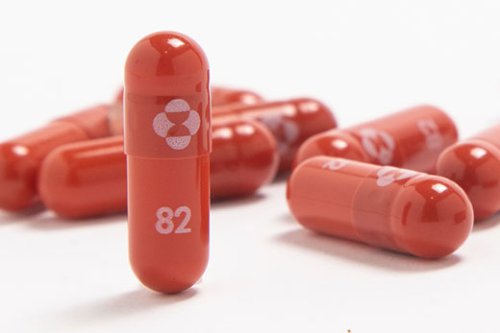
Drugmakers have asked the FDA for emergency authorization of molnupiravir, an antiviral pill that is used to treat mild to moderate COVID-19. Pharmaceutical companies Merck & Co. And Ridgeback Biotherapeutics announced Monday the submission of emergency use authorization to the Food and Drug Administration for molnupiravir, an antiviral drug that offers the promise that COVID-19 could soon be treated by a pill. Molnupiravir is used to treat mild to moderate adult cases of COVID-19 that are at risk of worsening to severe COVID-19 or hospitalization, according to the companies. Source 1o.
Merck Molnupiravir (Hover)
Merck Molnupiravir Health & Special Psychology
The FDA authorizes a second antiviral to fight COVID, a Merck pill that can be taken at home. A day after authorizing the first antiviral pill to treat COVID-19, the FDA approves Merck’s molnupiravir. The prescription medication is designed to stop the progression of COVID-19 from mild to severe symptoms in people at high risk. Though not as effective as Pfizer's pill, it is one more tool to help prevent illness from a COVID-19 infection. Source 8s.
 TOPIC: Merck Molnupiravir
TOPIC: Merck Molnupiravir Drug to control COVID-19 symptoms
Drug to control COVID-19 symptoms